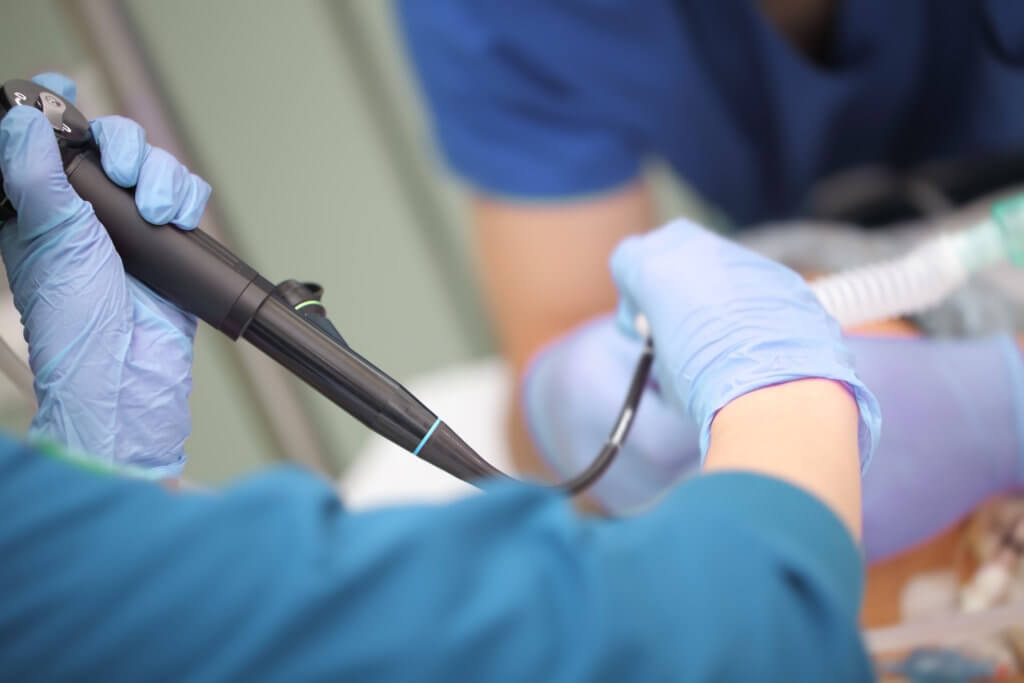
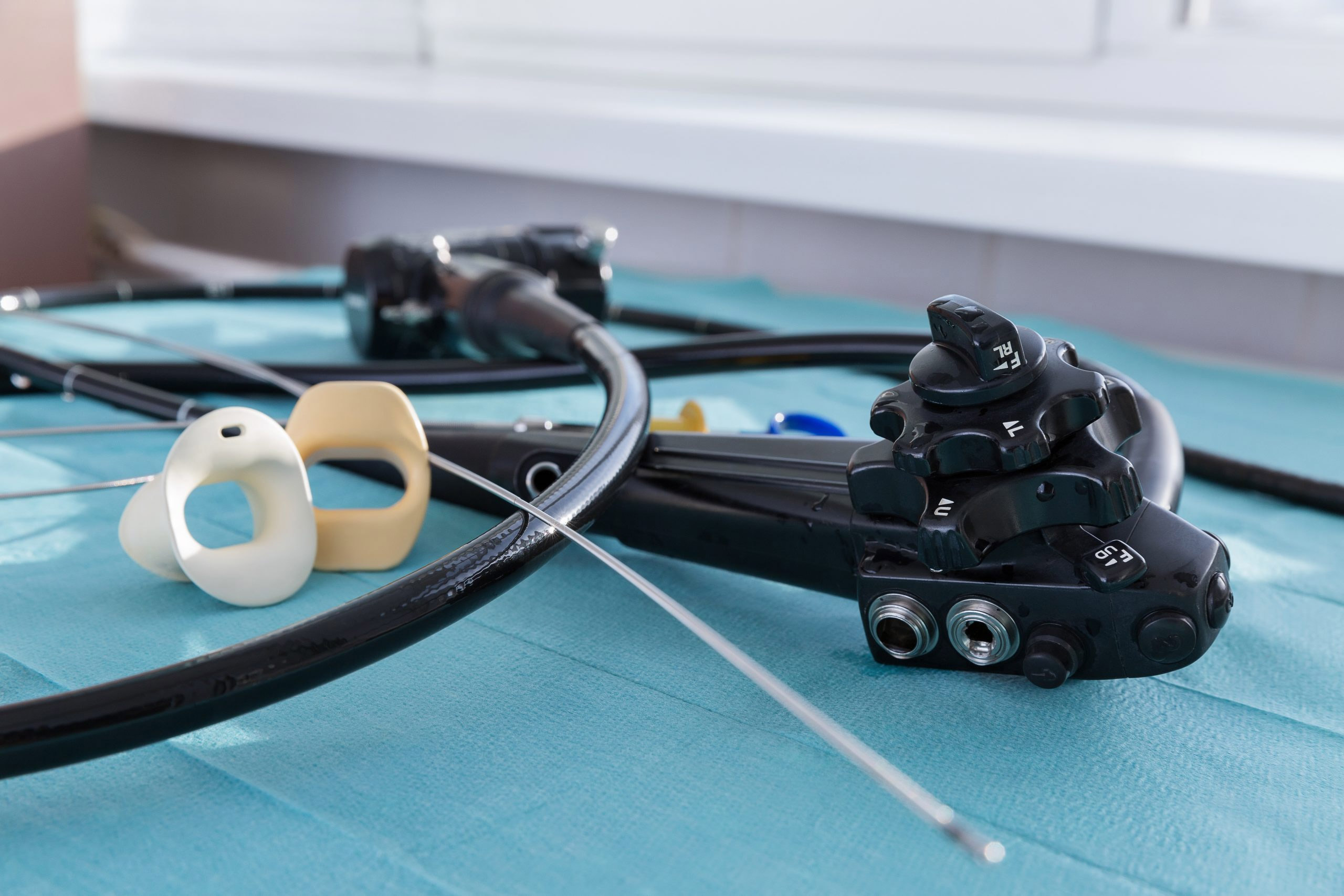
How can a hospital or clinic be certain an endoscope is truly patient-ready and safe? Are electronic systems effectively tracking every reprocessing task and step? Are there ways to enhance the cleaning, disinfection and storage assessments undertaken by an organization’s infection prevention and accreditation staff?
A study in the American Journal of Infection Control distills 10 years of endoscope audits by a “Clean Team” of health care practitioners into 13 key takeaways.
“Running an efficient endoscope auditing program is complex,” the study’s authors write. “Incorporating these key learning points into your own assessments will not only help you to improve compliance, but also build relationships with key team members.”
Flexible endoscopes are notoriously difficult to clean and disinfect, given their long, narrow channels and delicate nature. Manufacturers offer varying guidelines for cleaning, disinfecting and reprocessing scopes, and industry guidelines for reprocessing endoscopes include more than 100 steps. The process can take more than two hours to complete.
An estimated 500,000 bronchoscopies are performed annually so the risks of infection are relatively low. Research, however, suggests the true rate of infection transmission during endoscopy is vastly underreported due to poor surveillance or the absence of clinical symptoms.
The authors of this study—Rebecca Washburn, Eman Chami, Abigail Keskimaki, and Patricia Starr, regulatory accreditation and infection control professionals with Henry Ford Health System in Michigan—describe an endoscope-auditing journey that began in 2009 with a three-page document filled out by one person in about 30 minutes.
Over time, and out of necessity, that process grew into a standardized tool used at 27 sites (on the hospital campus as well as in offsite clinics) where endoscope reprocessing takes place. Henry Ford’s Clean Team re-evaluated its tools, processes, and goals and partnered with area managers, subject-matter experts, vendors, and staff.
A System Reprocessing Team was also born, comprised of supply chain, clinical engineering, employee safety, and accreditation staff. Today, auditing teams visit all locations with a standardized tool, review the entire workflow, and evaluate every step, from point of use through storage, according to the study.
The study’s authors offer perspective on 13 key learnings, including:
“By sharing our journey, we hope to help other auditors avoid some of the common pitfalls that we faced,” the authors write. “Our ongoing mission is to have a comprehensive auditing team that will ensure that every endoscope is patient ready, every time.”
Click here for more from the study, including the 13 main takeaways and conclusions on running something as complex as an efficient endoscope-auditing program.