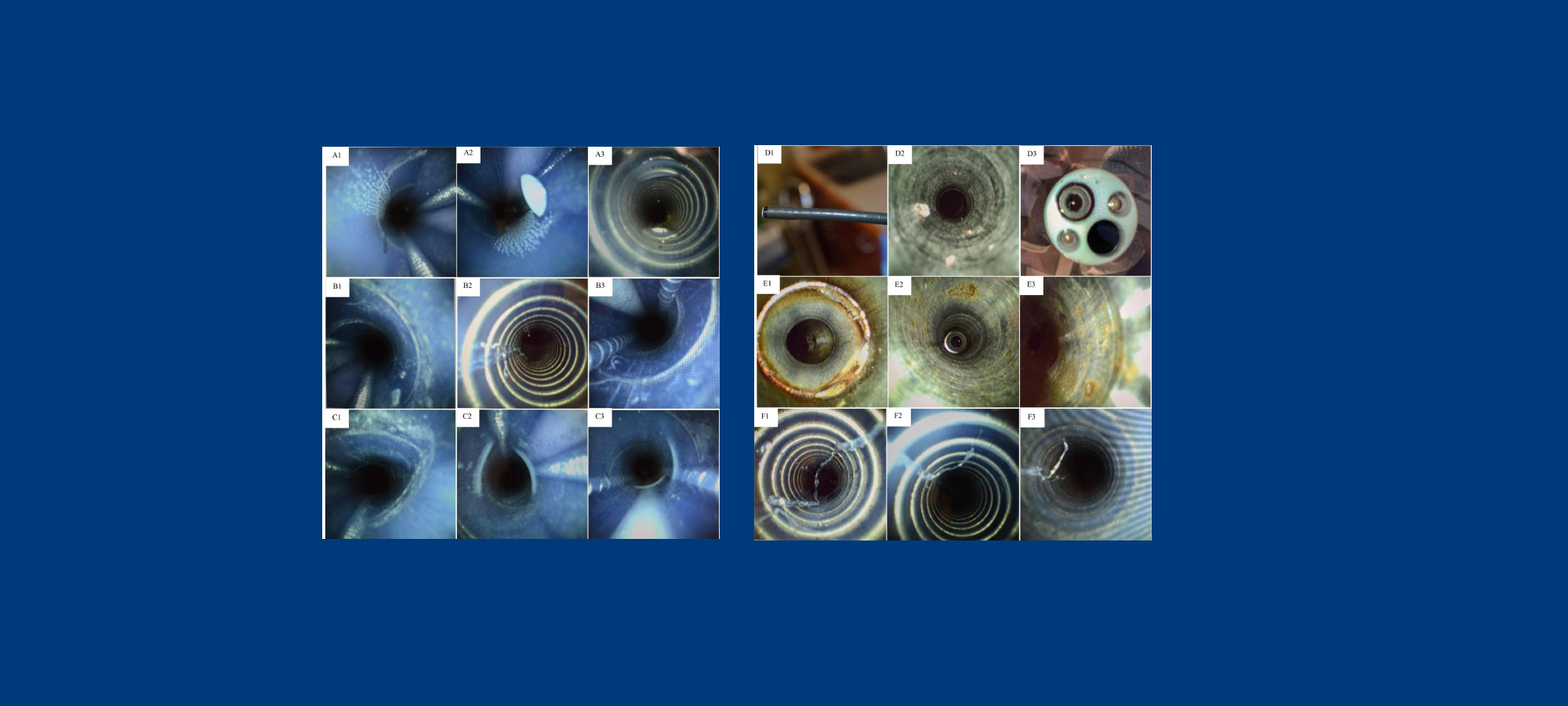
After University of Pittsburgh researchers found structural damage and particles in the medical center’s reusable flexible endoscopes, the facility stopped using devices with distal end protectors altogether.
That’s according to a study by Megan M. Wallace, a research technician at the university’s Graduate School of Public Health, and others, and published in the American Journal of Infection Control. Their research documented a variety of abnormalities and damage in reusable scopes from three different clinical areas.
A total of 42 endoscopes were cultured and 36 endoscopes were examined with a borescope. Positive microbial cultures were seen in 30 percent of the urological scopes, 28 percent of the bronchoscopes and 22 percent of the GI endoscopes examined, the study found.
Structural damage, foreign material (including red particles), and moisture within endoscopes were all detected by borescope examination.
“Borescope examination and microbial sampling should be used routinely to assure endoscopic safety,” the authors wrote. “The structural damages and the particles found in endoscopes resulted in timely repair and discontinuation of this type of distal end protectors in our facility.’
In a separate study by Cori L. Ofstead, an infection control expert, and others, visual inspection with magnification and borescopes identified actionable defects that could interfere with reprocessing effectiveness in 100 percent of the processed endoscopes tested. The research, published last year, was conducted in the GI endoscopy department of an urban hospital.
“The findings demonstrated that external inspection alone is insufficient to identify endoscopes with critical defects, and recent maintenance did not guarantee that the endoscopes were free from damage,” those researchers wrote.


