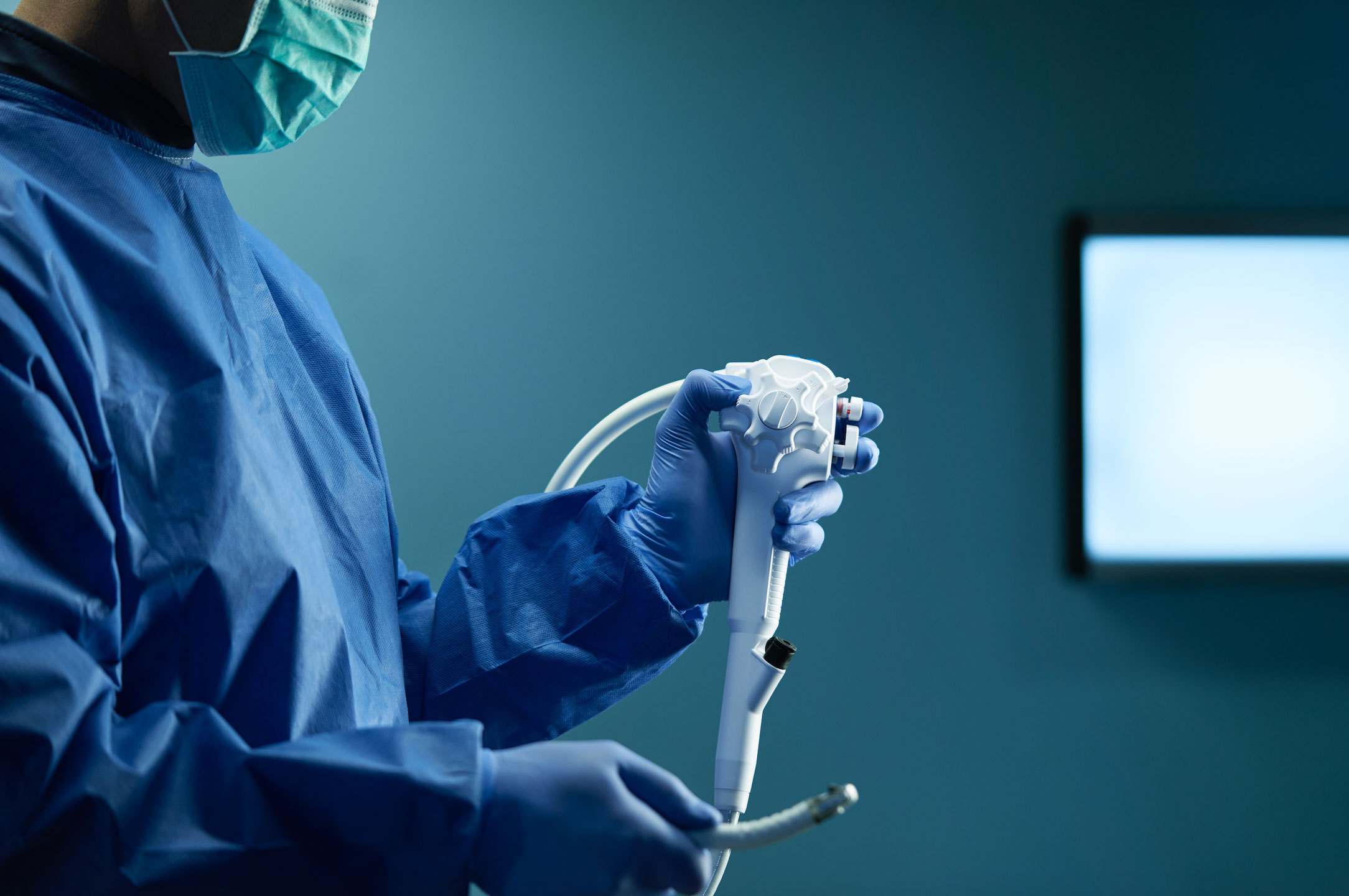
Advanced endoscopists can diagnose and therapeutically treat conditions in the pancreatic and bile ducts without the adverse risks of surgery thanks to duodenoscopes – flexible endoscopes used in endoscopic retrograde cholangiopancreatography, or ERCP.
The procedure is not without its risks, however, and duodenoscope-associated infections linked back to inadequate device reprocessing remain a concern for the U.S. Food and Drug Administration.
Innovations in the duodenoscope market, however, offer a promising outlook, according to a recent article in Medical Tubing + Extrusion. The publication called out five duodenoscope manufacturers driving advances in infection prevention, among them Denmark-based Ambu A/S and Boston Scientific Corp.
In December 2019 the FDA granted 510(k) clearance to the first single-use duodenoscope from Boston Scientific. The Ambu aScope Duodeno was cleared in July 2020.
“Ambu’s vision is to enhance patient safety, eliminate device-related cross-contamination, and simplify the procedure set-up,” Rasmus Kümmel Holtze, director of Ambu global product management in gastroenterology, told Medical Tubing + Extrusion.
Duodenoscopes are considered one of the most difficult endoscopes to clean due to their complexity. That’s particularly true because of the duodenoscope’s distal end, which includes an elevator mechanism for maneuvering accessories in the upper GI tract.
Contaminants can be lodged in the elevator mechanism and can pose a threat to patients in subsequent uses if not adequately removed.
Ambu CEO Juan Jose Gonzalez said in a recent podcast on DeviceTalks that technological improvements make single-use endoscopes as effective as their reusable counterparts while also providing infection prevention benefits.
“Every year, all the trends to move to single-use become stronger,” Gonzalez said.
Medical Tubing + Extrusion reports contamination rates for patient-ready duodenoscopes to be 5.4 percent for high-concern organisms such as E. coli and P. aeruginosa. A contaminated duodenoscope does not always lead to patient infection, but endoscope-associated infections are also likely underestimated due to poor surveillance and tracking.
In August 2019, the FDA recommended duodenoscope manufacturers switch to devices with disposable components or fully single-use models to help prevent patient infection.
In addition to the aScope Duodeno and the Boston Scientific scope, Medical Tubing + Extrusion named devices from Olympus America (disposable distal endcap), Pentax Medical (disposable elevator), and GI Scientific, LLC (distal end protective device).


