The ECRI Institute has broadened the scope of the threat posed by contaminated medical instruments on its newest list of top 10 technology health hazards.
The institute is training its lens on core infection prevention and control (IPC) practices in all healthcare settings, not just those where patients may come into contact with contaminated items, especially those that enter sterile tissue or the vascular system. Any acute care setting possibly not serviced by a central sterilization processing department—including dental offices and some medical offices—has the potential to expose patients to contaminated instruments, implants, or other items.
Infection risks from what it calls “sterile processing errors in medical and dental offices” ranks No. 3 on the ECRI Institute’s 2020 list.
“During IPC consultations in these settings, ECRI Institute has observed numerous oversights and improper actions associated with sterilization processes,” the report states. “While the prevalence of such failures is unknown, the potential exists for this to be an insidious, widespread patient safety risk.”
The report lists safety measures to undertake, including designating a qualified staff member or contractor to support office IPC practices. Training and periodic competency testing of benchtop sterilize operators is also recommended.
The list, now in its thirteenth year, identifies potential sources of danger that it believes warrant attention for the coming year. This newest analysis emphasizes “newly developing hazards and the migration of medical technologies to areas beyond the acute care setting”—which is why the list is topped by the risks associated with the use of surgical staplers. It’s a timely selection given media attention around the U.S. Food and Drug Administration’s push to reclassify these devices.
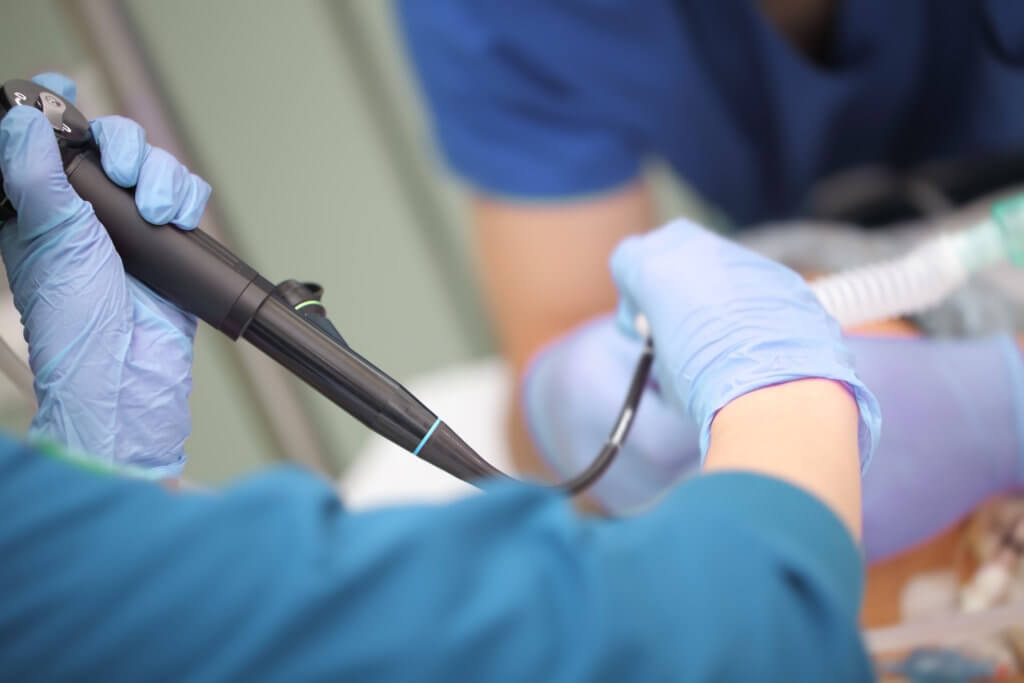
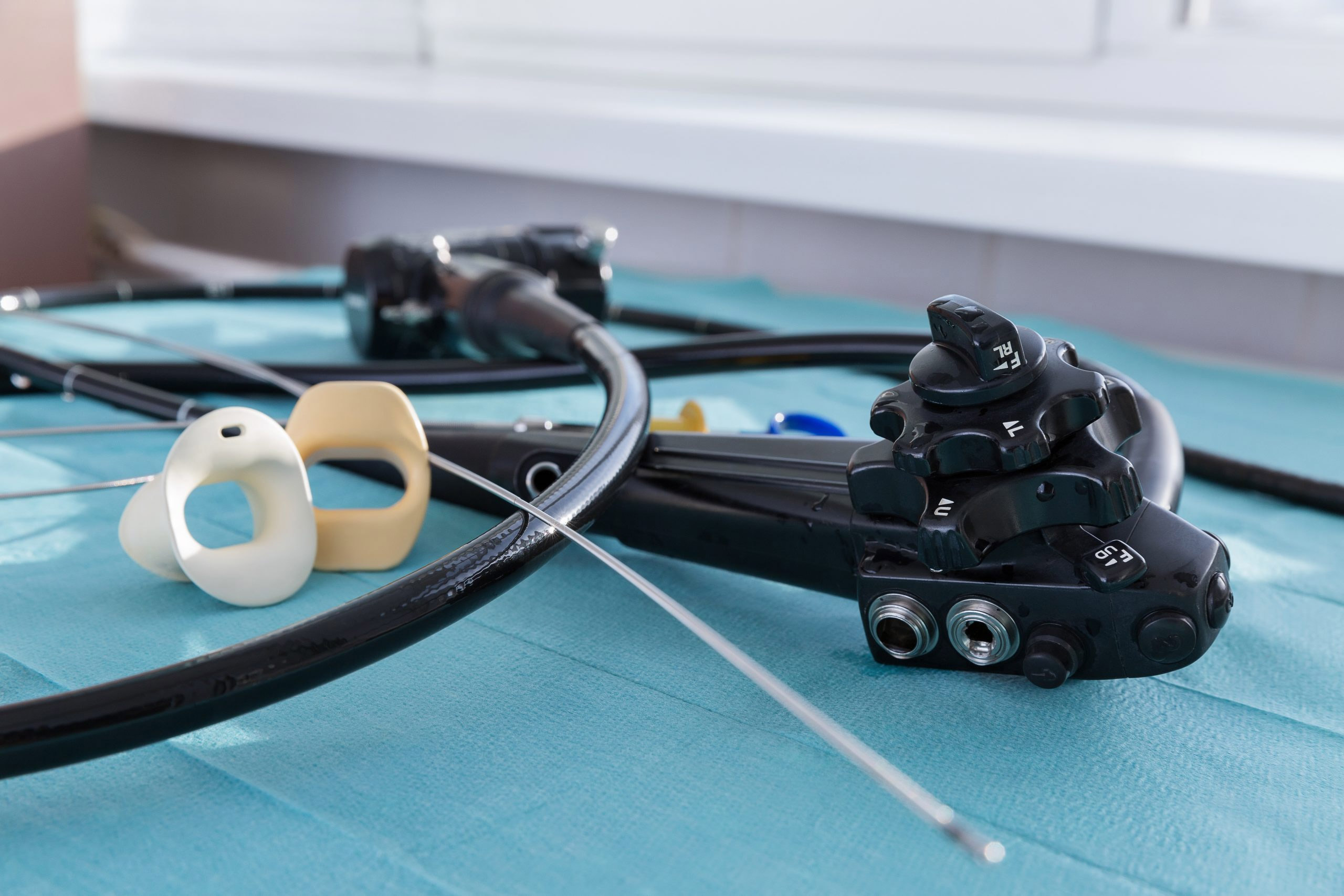
Flexible endoscopes have made the list every year since 2010, with cross-contamination topping their lists in 2010 and 2016.
Last year’s list, for the first time in more than a decade, shifted its focus from the threat posed by improperly cleaning endoscopes to the risk of recontamination due to improper handling after an endoscope has been cleaned. That technology hazard came in at No. 5.
Flexible endoscopes are notoriously difficult to clean and disinfect, given their long narrow channels and delicate nature. Manufacturers offer varying guidelines for cleaning, disinfecting and reprocessing scopes, and industry guidelines for reprocessing endoscopes include more than 100 steps. The process can take more than two hours to complete.
As the ECRI Institute recognized on last year’s hazards list, the threat of recontamination remains even after endoscopes are cleaned and disinfected. Endoscopes must be handled and stored properly after reprocessing and before reuse to avoid recontamination.
Bronchoscopes are one of the most commonly used instruments in a hospital, enabling doctors to see the airways to the lungs and perform interventions such as lung biopsies or removing pus and tumors. Reusable bronchoscopes also pose a high risk of cross-contamination.
For more information about industry guidelines regarding endoscope drying and storage, visit www.bedsidebronchoscopy.com/guidelines.