There is virtually no risk of infection transmission associated with noncritical medical items and even critical devices have been rarely linked to such cases.
The exception, according to a recent major article published in the American Journal of Infection Control, is semicritical items. Semicritical instruments are those that come into contact with mucous membranes or non-intact skins, according to the paper’s authors, Dr. William A. Rutala and Dr. David J. Weber, based out of the University of North Carolina in Chapel Hill.
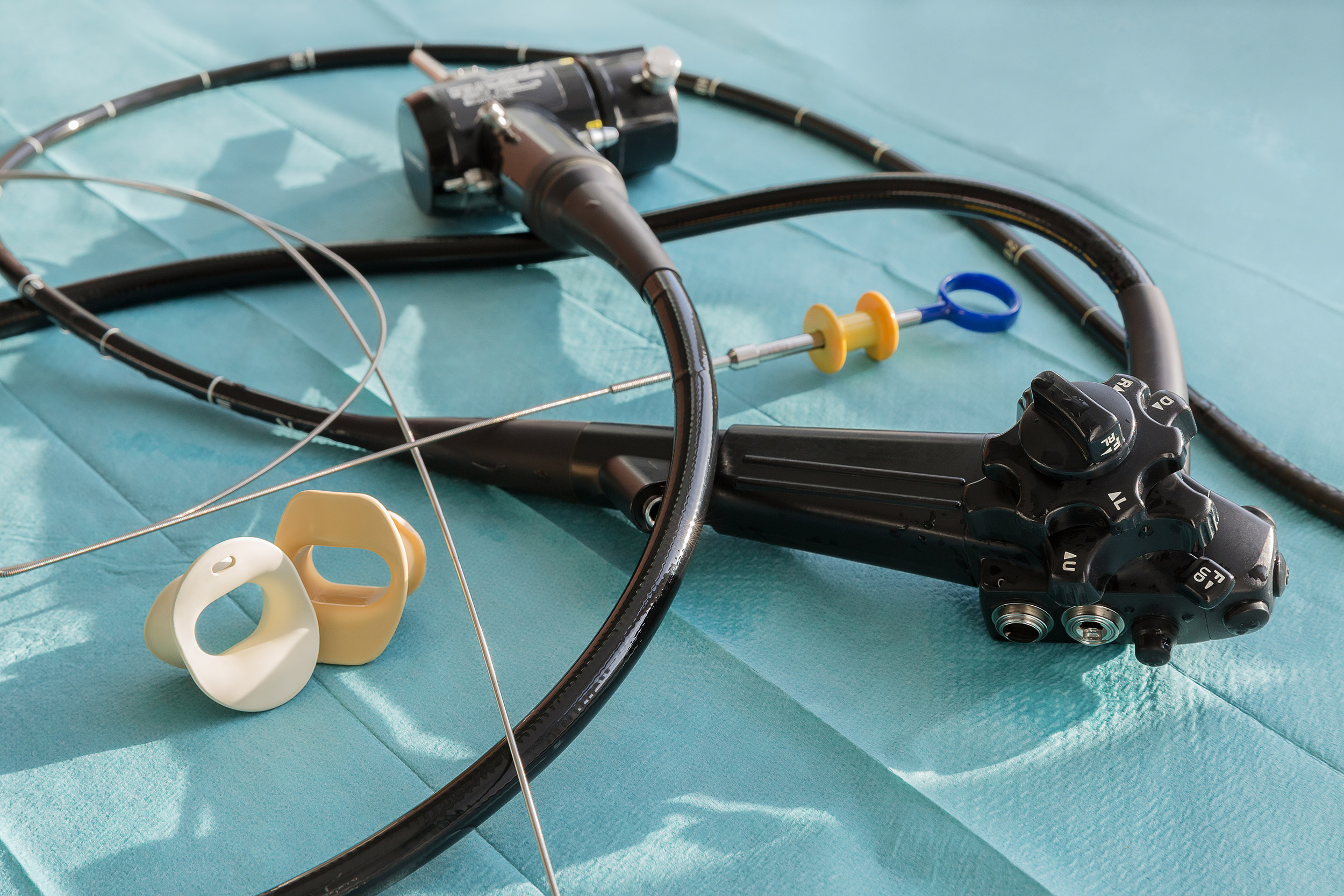
These devices include gastrointestinal endoscopes, bronchoscopes and urological endoscopes, among others. Semicritical items require high-level disinfection (HLD) at minimum, but the paper explores the compelling rationale for transitioning away from HLD to sterilization, along with the many issues associated with reprocessing these instruments.
“Since semicritical equipment has been associated with reprocessing errors that result in patient look back and patient notifications, it is essential that control measures be instituted to prevent patient exposures,” the authors write. “Before new equipment (especially semicritical equipment as the margin of safety is less than that for sterilization) is used for patient care on more than one patient, reprocessing procedures for that equipment should be developed.”
The article includes instructions for rinsing, drying and storage of reusable endoscopes. It also examines the benefits of borescope utilization.
“While endoscopes represent a valuable diagnostic and therapeutic tool in modern medicine and the incidence of infection associated with use has been reported as low, more healthcare-associated outbreaks have been linked to contaminated endoscopes than to any other medical device,” they write. “In fact, over the past several years alone there have been greater than 25 outbreaks of multidrug-resistant organisms (MDROs such as CRE) in major hospitals in the United States and the world that have killed dozens of patients and caused morbidity in hundreds more.”
Such outbreaks have been primarily linked to duodenoscopes, the authors write, and have occurred despite correct cleaning and HLD. The concern, they add, is whether current reprocessing guidelines are adequate.
Sterilization has been most successful at addressing reprocessing concerns. The authors additionally stress the importance of strict adherence to national reprocessing guidelines and manufacturers’ instructions for use.
In recent years, the U.S. Food and Drug Administration has released safety communications involving bronchoscopes, urological endoscopes and duodenoscopes — in which it calls for transition to partially or fully disposable alternatives.
“Fully disposable duodenoscope and bronchoscopes eliminate the potential for infections caused by ineffective reprocessing,” the authors write. “Current observations suggest that the majority of ERCP procedures can be safely and effectively performed using single-use duodenoscopes.”
Other semicritical items explored in the article include:


