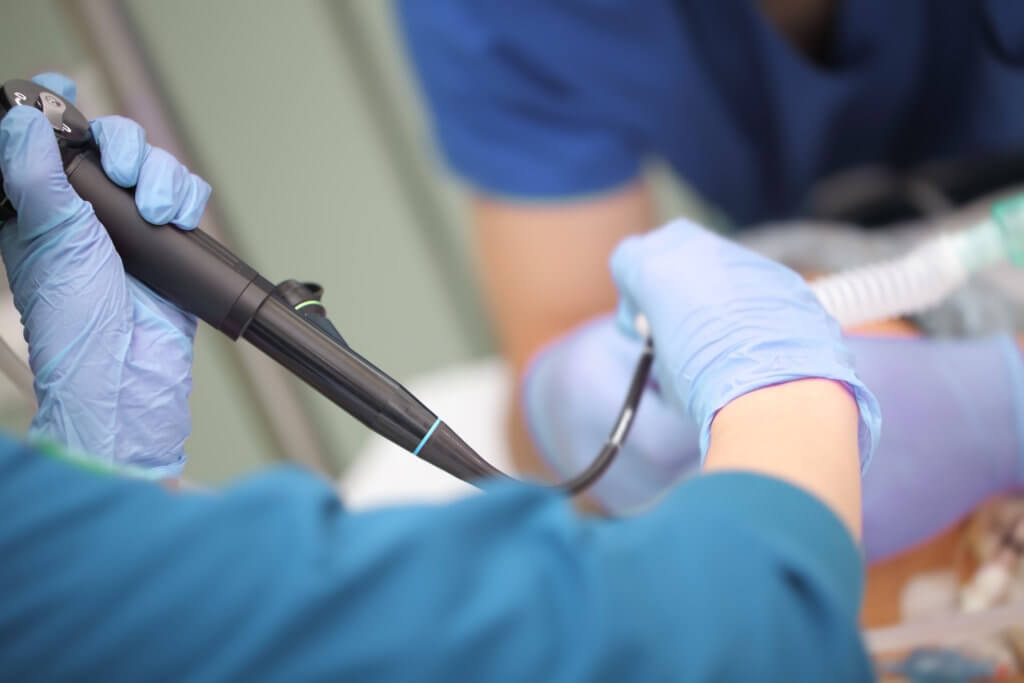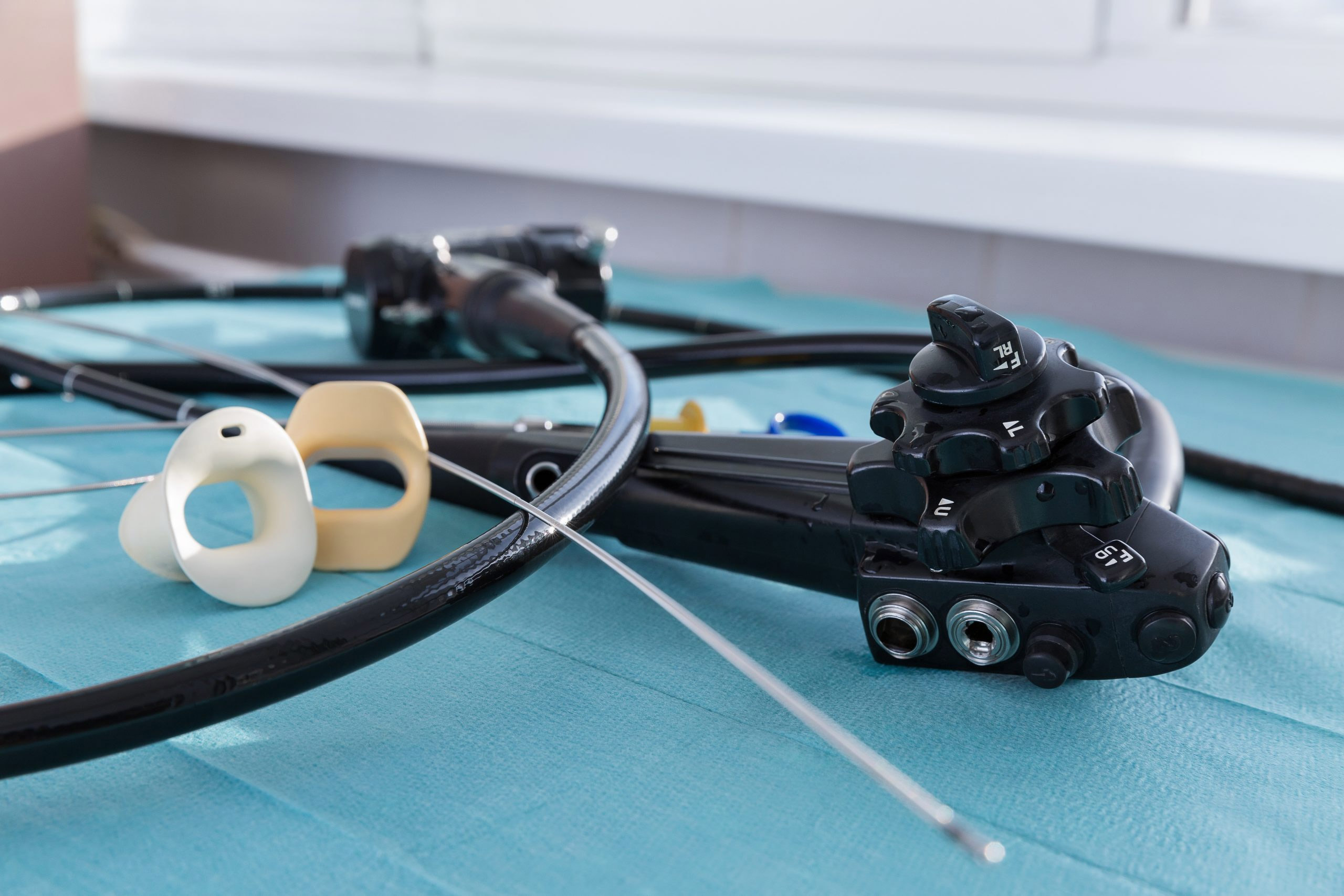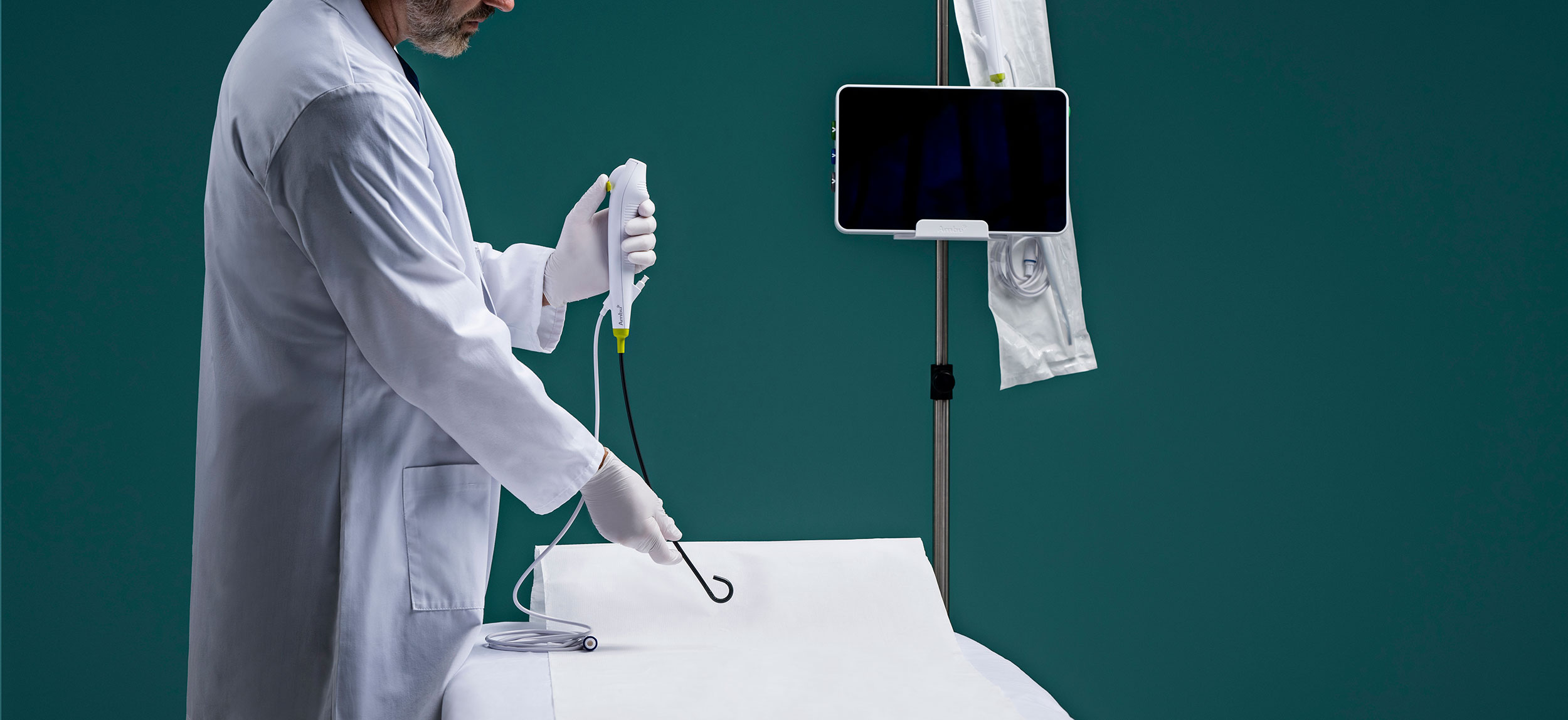
Which scopes are best suited for urology outpatient facilities, single-use or reusable cystoscopes and ureteroscopes?
That is the question posed in a recent article in Outpatient Surgery, an AORN publication, which examined whether urologists should continue to invest in reusable devices that require reprocessing or transition to single-use scopes.
The choice between reusable and disposable scopes sometimes comes down to economics. Outpatient Surgery noted that a high-volume center might find reusable scopes more economical, while facilities with lower urologic volume might find single-use scopes a more cost-effective option.
However, Dr. Brad D. Lerner, president of Chesapeake Urology Associates, suggests that high-volume urology centers might find single-use scopes more affordable if Medicare chooses to cover their cost fully.
This possibility is backed by the Centers for Medicare and Medicaid Services (CMS) approval in January 2023 of an Incremental Device Reimbursement (code C1747) for certain ureteroscopes and manufacturers.
“If there truly is going to be reimbursement for at least the cost of these single-use scopes, one would question the need for future purchase of fiber-optic or digital scopes that will require processing, storage, and preventative maintenance and service,” said Lerner.
For facilities with lower urologic volume, single-use scopes present a compelling option, said Outpatient Surgery. They eliminate the time, personnel, and expense needed for reprocessing, and remove concerns about cross-contamination.
There are also infection prevention considerations with this choice. In April 2021, the U.S. Food and Drug Administration announced it was investigating “numerous” medical device reports (MDRs) describing patient infections and other possible contamination issues possibly associated with reprocessed urological endoscopes.