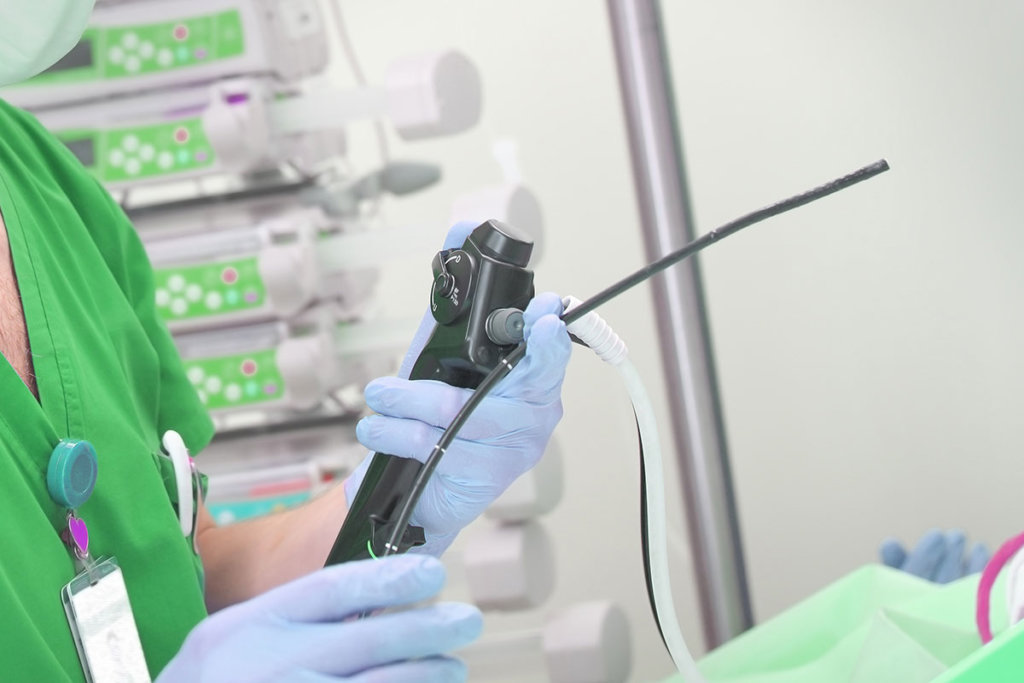
The Food and Drug Administration’s announcement that hospitals and endoscopy facilities transition to duodenoscopes with innovative designs to boost patient safety has stoked the market’s appetite for disposable scopes. But producing one has proved challenging for device manufacturers.
These companies must strike a tricky balance between disposability and functionality—and they must do it with cost-effectiveness in mind. While manufacturers Fujifilm and Pentax have received FDA clearance for scopes with disposable endcaps, neither has managed to date to bring a fully-disposable scope to market, nor have they yet announced plans to do so.
Two other device manufacturers plan to debut fully-disposable devices in the near future. Danish manufacturer Ambu anticipates the U.S. launch of its single-use scope in 2020.
Boston Scientific’s own disposable model won FDA clearance in December 2019. The company plans a "limited market release" in early 2020. Another issue is the viability of existing reprocessing techniques. It falls on manufacturers to develop reliable instructions for the cleaning and maintenance of their devices. The recent FDA announcements—along with calls to action from gastroenterological advocacy societies like AGA, ASGE, and SGNA—suggest that, at the least, a major revision of reprocessing instructions is overdue.
“The AGA Center for GI Innovation and Technology continues to engage with stakeholders around this issue and is committed to collaborating on a solution to ensure zero device-associated infections,” AGA officials said in a February 2018 statement supporting new government scope safety protocols.
Read more:
The instructions for manually cleaning scopes can total more than 100 distinct steps. And with such complex procedures, errors tend to accumulate. Fujifilm’s usability studies found, for example, that 67 percent of technicians failed to properly scrub one model’s forceps elevators. On an Olympus instrument, 82 percent of technicians did not complete an elevator brushing task as described in the manual.
It’s not clear if introducing disposable components would resolve such usability problems. Infection-prevention consultants point out that scopes’ narrow tubing and small, moving parts will remain difficult to visualize and clean. Short of being fully disposable, scopes with new parts may not have a meaningful impact on technician error.
After manual cleaning, manufacturers’ instructions call for disinfection to kill off any remaining microorganisms. Postmarket surveillance indicates, however, that current disinfection techniques (using automated endoscope reprocessors) fall short of expectations. The FDA has requested that manufacturers work to improve disinfection processes. But here, manufacturers run into several challenges.
For one, duodenoscopes don’t weather today’s disinfection techniques very well. Scopes are heat-labile. High temperatures can crack the glass and metal components of duodenoscopes’ tips. High-heat sterilization, then, isn’t a viable option for duodenoscopes.
Low-temperature terminal sterilization techniques, such as those using hydrogen peroxide plasma or ethylene oxide, come with their own limitations. Although effective on antimicrobials, these chemicals degrade duodenoscopes’ soft components and adhesives, eventually rendering them unusable. The Environmental Protection Agency, moreover, has also concluded that emissions from the production of these chemicals may be hazardous for the environment.
The FDA has issued a challenge to develop new sterilization techniques, but there’s no telling when a solution might avail itself to device manufacturers. For now, they will have to either totally redesign scopes to be more amenable to sterilization, or develop fully-disposable scopes, which needn’t be sterilized at all.


