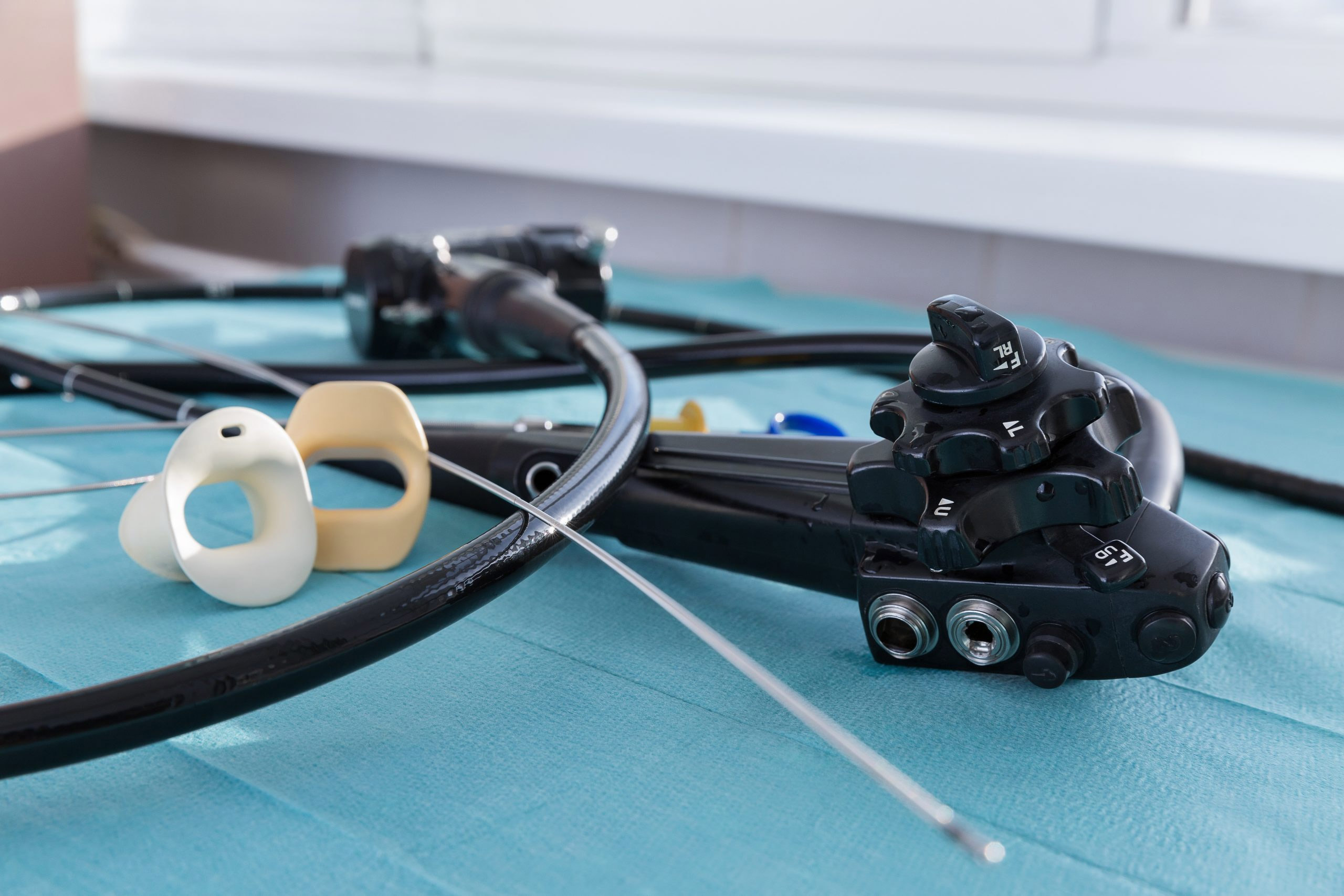
Medical device manufacturer Karl Storz has initiated a voluntary recall and issued an urgent field safety notice instructing customers to discontinue high-level disinfection and, in some cases, liquid chemical sterilization of some of its flexible urological endoscopes.
The affected devices are single-channel endoscopes with an attached T-Luer intended for diagnostic and therapeutic purposes.
The April 1 notice lists 28 products, including flexible cystoscopes and ureteroscopes. Customers without access to “an appropriate sterilization method” should discontinue the use of these endoscopes and return them, the company said.
Testing of the manual high-level disinfection process showed that required efficacy levels were not reached. In response, Karl Storz removed manual high-level disinfection and automated high-level disinfection via an automated endoscope reprocessor. Liquid chemical sterilization was removed for certain products.
“As the efficacy of the manual high-level disinfection process cannot be assured for the affected products, there is a risk that the patient may be exposed to a higher risk of infection,” the notice reads. “The use of a flexible endoscope that is incompletely reprocessed with an ineffective disinfection or sterilization cycle has a potential to transmit a patient infection.”
The U.S. Food and Drug Administration issued a letter April 4 to healthcare providers detailing the recall and underscoring the reprocessing recommendations. The agency said it will continue to monitor reports of patient infections or contamination issues and work with manufacturers on reprocessing methods and instructions.
The FDA’s letter follows an announcement in April 2021 that the agency was investigating “numerous” medical device reports describing patient infections and other possible contamination issues possibly associated with reprocessed urological endoscopes.
The Karl Storz notice includes seven actions for customers to take to mitigate risk. The company is asking to be notified by customers if any adverse events or quality problems associated with the use of affected endoscopes occur. Adverse events or quality problems may be reported to FDA’s adverse event reporting program, called MedWatch.