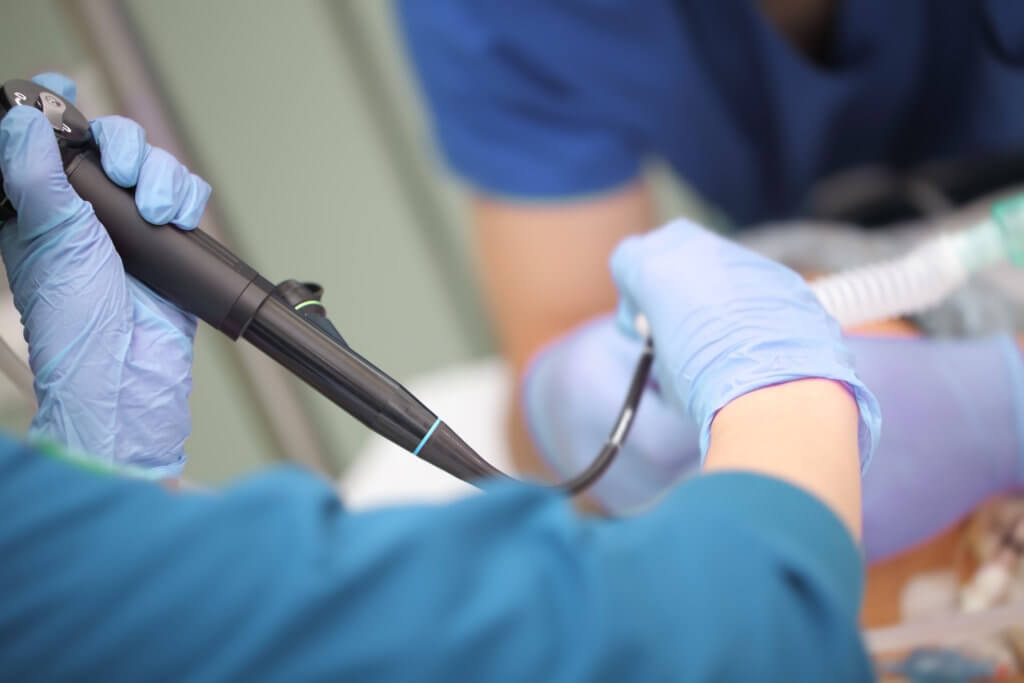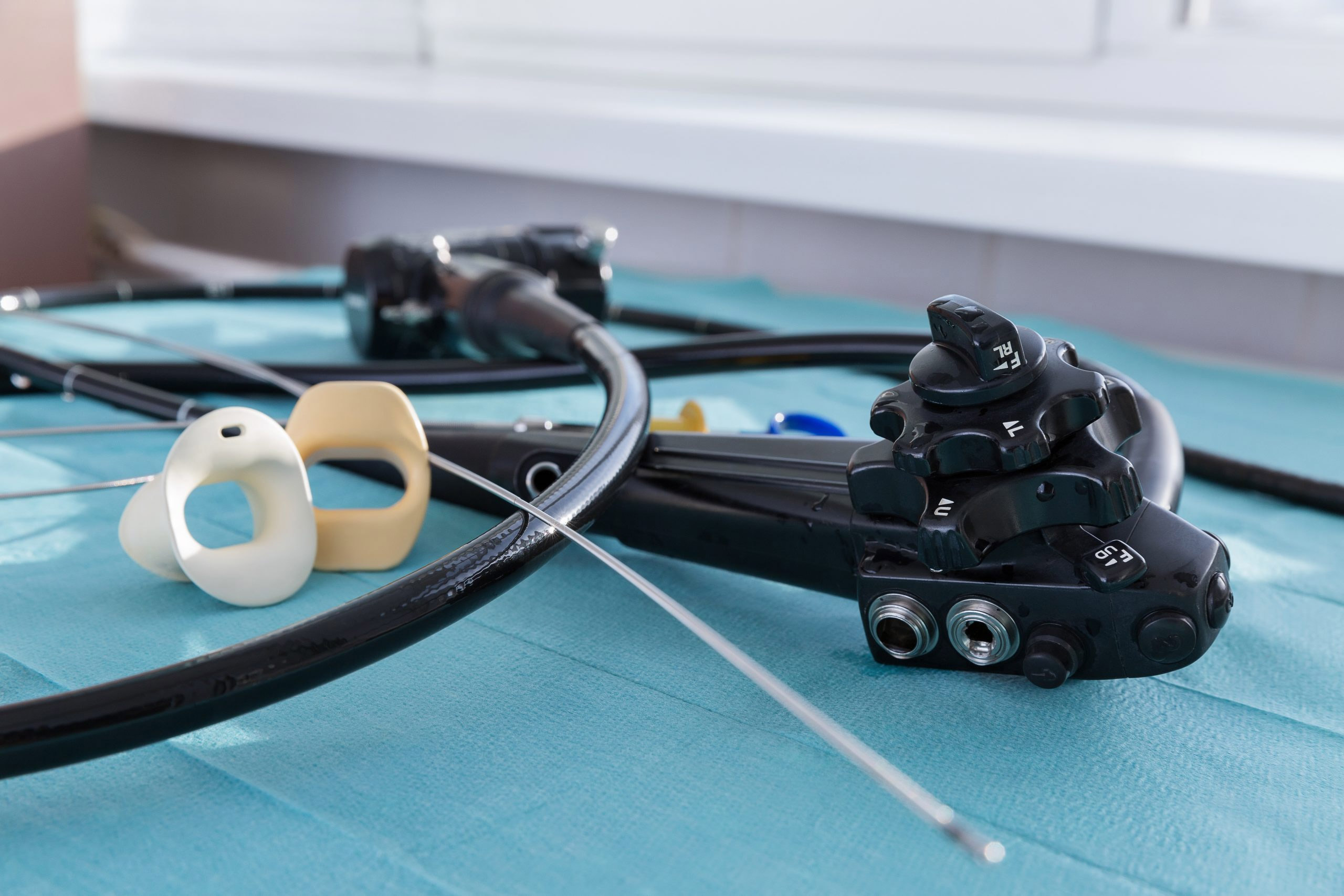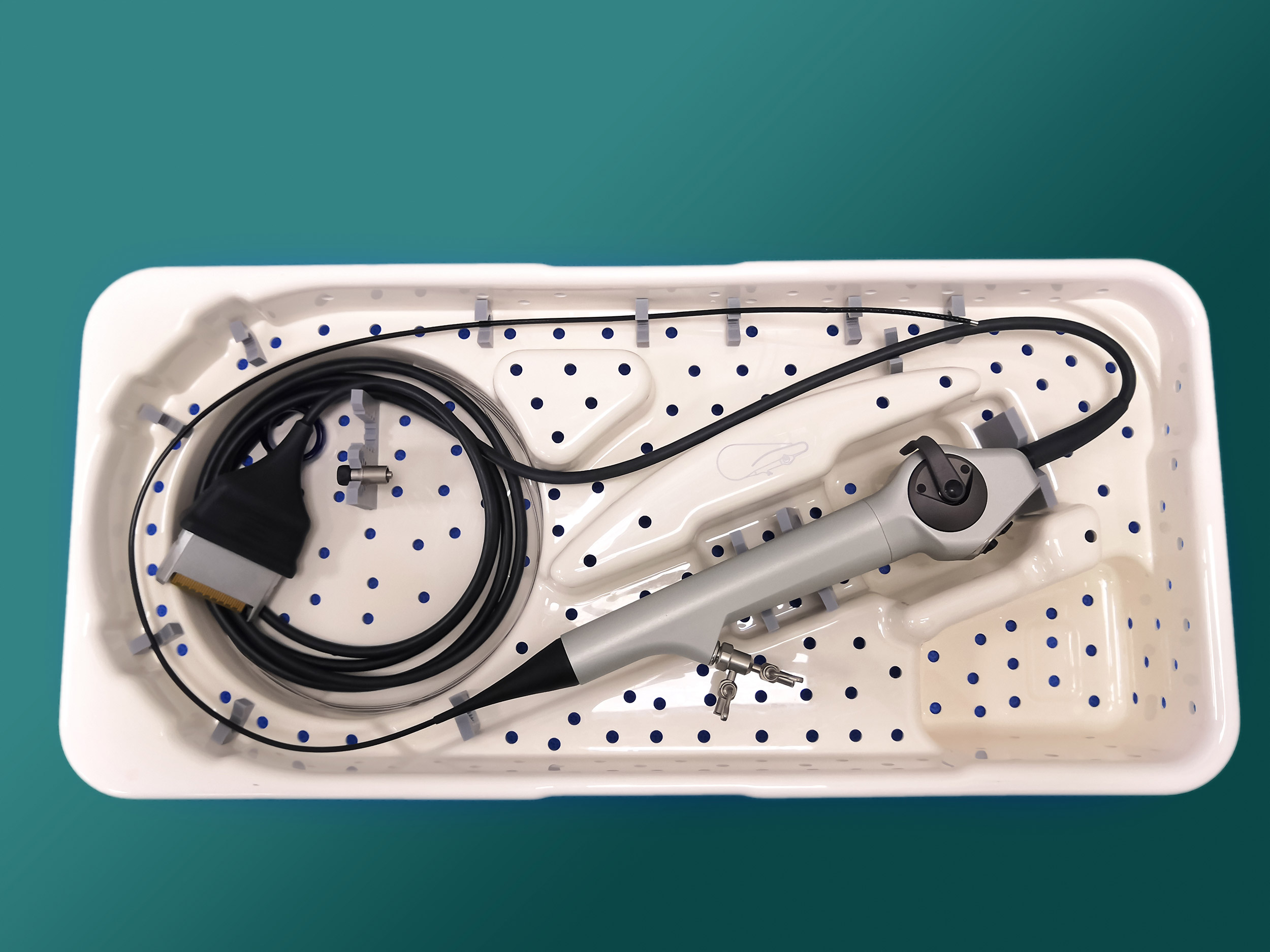
More than 100 new medical device reports (MDRs) on flexible ureteroscopes have been filed with the U.S. Food and Drug Administration since the agency released a letter to healthcare providers last spring announcing its investigation into numerous MDRs involving reprocessed urological endoscopes.
This was among the key findings presented in a recent OR Manager webinar featuring Cori Ofstead, an epidemiologist with more than 25 years of research experience.
Since the FDA issued its letter on April 1, 2021, 105 MDRs have been filed with the agency on flexible ureteroscopes. This includes more than 35 in both August and October of 2021. These reports included:
“This indicates that the problems haven’t gone away, and possibly, more people are filing reports since they heard about this risk and they were urged by the FDA to submit reports,” Ofstead said.
In addition to filing MDRs for adverse events involving urology scopes, the FDA also urged manufacturers to:
Ofstead offered a detailed breakdown of the complex designs of ureteroscopes, which are significantly smaller than many other endoscopes and therefore require inspection under magnification after each cleaning. She also suggested quality management programs worth implementing. These included considering the role of single-use ureteroscopes.
Ofstead leads a multidisciplinary team that specializes in designing and conducting real-world studies to validate healthcare guidelines, treatments, and product claims.